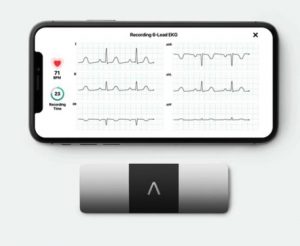
In this episode we will discuss AliveCor announced Thursday, July 8 it received 510(k) clearance by the U.S. Food and Drug Administration for healthcare professionals to use the KardiaMobile 6L device to calculate patients’ QTc interval. We will talk about the Kardia device and explain why this new announcement is important to those who have an interest in their heart health. More info on the device is at https://alivecor.com.
Podcast: Play in new window | Download